News
Sino Biopharm Was Selected as one of the Top 20 Competitive Chemical and Pharmaceutical Enterprises in China in 2022
Release time:2022-10-28
Recently, the series of "2022 China Top 100 Biopharmaceutical List" launched by Times Media Group has been launched one after another, and on October 24, its sub-list "Top 20 Competitive Chemical and Pharmaceutical Enterprises in China in 2022" was released, and Sino Biopharm was ranked fourth.
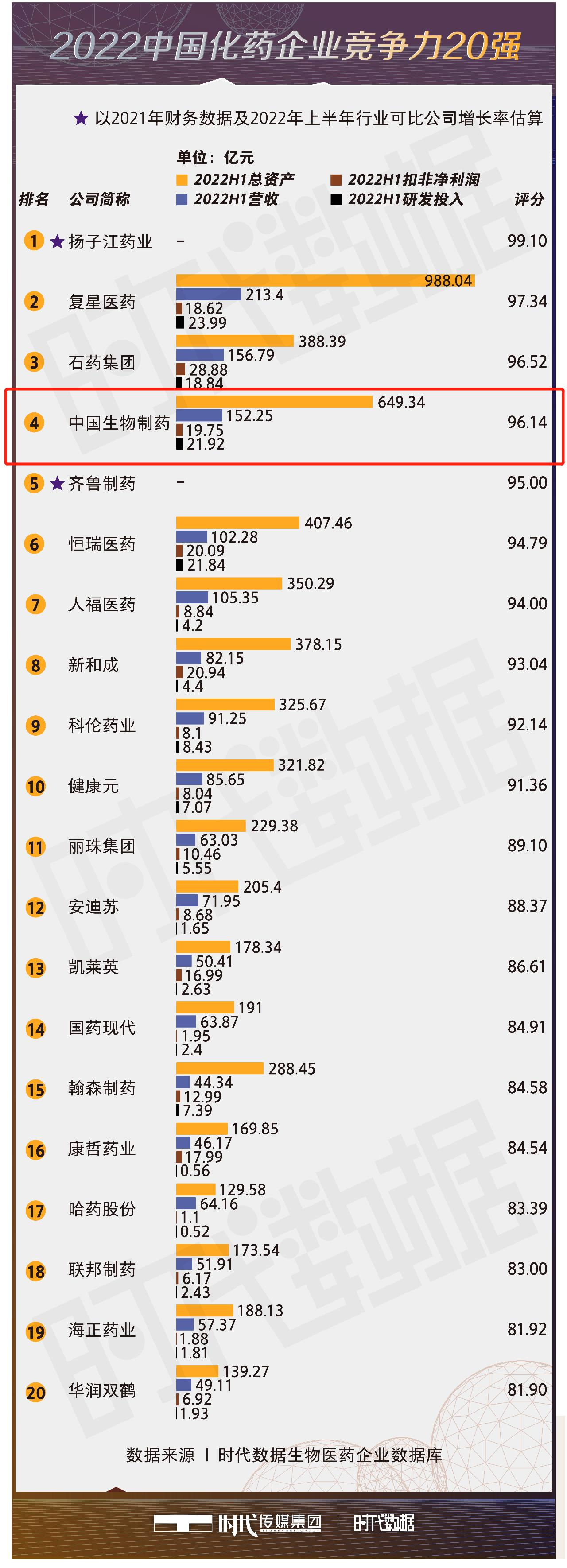
Based on the "Times Data Biopharmaceutical Enterprise Database", Times Data analyzed the operating revenue, net profit after deductions, R&D investment, total assets, cash flow from operating activities, monetary funds, ending inventory, total number of employees and other data of major chemical companies in the first half of 2022 through big data analysis, and made a comprehensive score.
The chemical pharmaceutical industry has always been the most important part of China's biopharmaceutical industry. As a well-known pharmaceutical company that has been deeply involved in the biopharmaceutical field for many years, Sino Biopharm has been increasing the innovation and R&D of chemical drugs, combining the R&D concepts of independent innovation, joint development and creation of generic development to continuously improve the level and speed of R&D. The investment in R&D in the first half of 2022 increased to 2.19 billion yuan, up 16.5% year-on-year and reached a record high of 14.4%. In the first half of 2022, R&D investment increased to RMB2.19 billion, up 16.5% year-on-year, accounting for a record high of 14.4%. Revenue from innovative drugs is expected to reach 24% in 2022 and is expected to exceed the RMB10 billion mark in 2023, further increasing its share. 2030 will see the Group's revenue reach HK$100 billion, of which innovative drugs will account for 60%.
Since 2022, the company has independently developed a number of Class 1 innovative drugs approved for clinical trials, such as a new target and a new mechanism of oral small molecule drugs, TDI01, which was listed as a major new drug creation in the National "13th Five-Year Plan", and in addition to the previously approved clinical trials for idiopathic pulmonary fibrosis in China, the company was also approved for clinical trials for pneumoconiosis and anti COVID-19 this year. In addition, a number of Class 1 innovative drugs such as FHND5071, TQB2930 and TQC2938 injection were also approved for clinical use this year. Meanwhile, TQ-B3101 capsule and TQ-B3139, the Group's oncology class 1 innovative drugs, will also be filed for marketing in the first half of 2022.

While stepping up its own R&D, Sino Biopharm is also introducing more innovative products through M&A investment and external introduction.
For example, its subsidiary CTTQ recently signed an agreement with Inventiva, a French biotechnology company, under which the company will acquire the exclusive license rights to develop, manufacture and commercialize lanifibranor, a non-alcoholic steatohepatitis treatment drug, in mainland China, Hong Kong, Macau and Taiwan (Greater China), which is expected to fill the gap in the domestic non-alcoholic steatohepatitis treatment field. In addition, since 2022, Sino Biopharm has also entered into collaborations with a number of domestic and international pharmaceutical companies, including Anyuan Pharmaceuticals and XtalPi, in order to develop or commercialize a number of innovative and differentiated products.
Sino Biopharm has long held the vision of "focusing on innovation, serving patients, and becoming a leading global pharmaceutical company", and has been vigorously promoting innovation and R&D in pursuit of its mission of "improving the quality of life and maintaining the dignity of life". Currently, Sino Biopharm has a rich pipeline of four major areas: antineoplastic, surgical/analgesic, hepatology and respiratory, with three innovative drugs approved for marketing, two innovative drugs already in production, over 50 innovative products in various clinical stages and over 60 innovative drug candidates in preclinical stage.
At the same time, the company has a number of internationally leading new technology platforms and a specialized and fully capable R&D team, laying a solid foundation for the continuous harvest of innovative R&D results. In the future, Sino Biopharm will continue to deepen its innovation, focus on key disease drugs, and continuously improve drug accessibility from multiple dimensions to help the patients receive treatment in the most convenient and economical way. We will contribute to the improvement of quality of life and the health of all people.