News
Sino Biopharm Released 2021 ESG Report and Focus on Strengthening the Construction of"Responsible Sino Biopharm"
Release time:2022-05-31
On May 31, the Sino Biopharm 2021 Annual Environmental, Social and Governance (ESG) Report was released.

As an innovation-led biopharmaceutical company, in 2021, Sino Biopharm will take "maintaining the dignity of life and improving the quality of life" as its development vision, adhere to the concept of "technology creates value" and the values of "benefit the country, benefit the people and benefit the enterprise". In the year 2021, Sino Biopharm will take "maintaining the dignity of life and improving the quality of life" as its development vision, adhere to the concept of "science and technology create value", adhere to the values of "for the country, for the people and for the enterprise", fully respond to the national "14th Five-Year Plan", actively participate in the construction of a healthy China and contribute to the "double carbon" goal.
The Group clearly focuses on the construction of "Responsible Sino Biopharm", guiding the deep integration of ESG concept and management operation from a strategic perspective, continuously improving the level of ESG governance, ensuring that the Group achieves high-quality sustainable development, and contributing to human health and social harmony.
| Driven by innovation and promote the health for all
Sino Biopharm is oriented to clinical needs, patient needs, and driven by innovative R&D, and is committed to eliminating disease and pain, creating a healthy and better life for the people, and contributing to the health for all.
1、Increase investment in innovation, and carry out domestic and overseas industry cooperation
In 2021, Sino Biopharm continued to invest in innovation and research field and achieve comprehensive improvement in innovative technologies, R&D capabilities, personnel reserves and equipment iterations, etc. In 2021, the Group invested RMB 3.82 billion in R&D, accounting for 14.2% of revenue, an increase of 34.5% year-on-year. From 2019 to 2021, the compound growth rate of R&D investment is 20.1%.
2021 is also the year of vigorous expansion of Sino Biopharm's innovation frontier layout: In June, Sino Biopharm signed a strategic cooperation agreement with Shanghai Huangpu District and Minhang District, announcing that its China headquarters and global innovation R&D center will be located in Shanghai; in August, it officially launched its innovation transformation project, X-LAB, to accelerate the layout of its global strategic network.
Meanwhile, in 2021, Sino Biopharm made collaborations with overseas and domestic companies, including the Group's strategic investment in Treadwell Therapeutics; its subsidiary CTTQ entered into an exclusive strategic partnership with Genetron, thereby benefiting more patients.

CTTQ signs exclusive strategic cooperation agreement with Genetron
2、 Improve the accessibility of medicines
Sino Biopharm has always been committed to making medicines accessible to more patients and more diseases, and in 2021, we are continuing our work on pharmaceutical inclusion in four main directions: expanding therapeutic areas, reducing patients' burden, expanding channel coverage and development on drugs for rare disease.
Expanding therapeutic areas: We continue to increase our R&D investment in key diseases such as tumor and cardiovascular diseases, and continue to launch new products with definite efficacy. We also focus on other disease areas in addition to our key diseases. In addition to oncology and cardiovascular diseases, our products are also involved in various fields such as orthopedics, liver disease, respiratory system, analgesia and parenteral nutrition.
Alleviating patients' burden: In 2021, the percentage of listed drugs included in the Group's pooled procurement reached 41.49% of the varieties that were finally awarded through negotiations, and the prices of awarded products dropped significantly, alleviating the financial burden for more patients. We actively participate in medical insurance negotiations to benefit people's livelihood with better prices, while actively promoting the development of generic drugs and accelerating the consistency evaluation of generic drugs to provide more choices for patients.
Expanded channel coverage and medication knowledge: In December 2021, the Group's subsidiary Chia tai Qingjiang held an official launch ceremony for the "Ya Miao Health" app to create a professional health management solution that combines health management and health data into one. In addition, we also provide assistance to patients who have questions about medication information by opening a 24-hour 400 telephone and online consultation, informing patients to follow medical advice on medication use.

Jiangsu Chia tai Qingjiang "Ya Miao Health" APP official public test launch ceremony
Development on drugs for rare disease: We insist on rare disease research and continue to develop and manufacture rare disease drugs to provide more choices for rare disease patients. Currently. We have marketed edaravone sodium chloride injection for the treatment of acromegaly, and are developing recombinant human clotting factor VIII for injection and recombinant human clotting factor VIIa for injection for hemophilia, and Nintedanib Esilate Soft Capsules for idiopathic pulmonary fibrosis.

Jiangsu Chia tai Fenghai Participates in the First Rare Disease Forum for Amyptrophic lateral sclerosis
| Put people first, build a healthy ecology together
Sino Biopharm adheres to the principle of putting people first and attaches great importance to subjects, customers, corporate employees as well as consumers and investors.
1、Center around the whole life cycle to ensure public drug safety
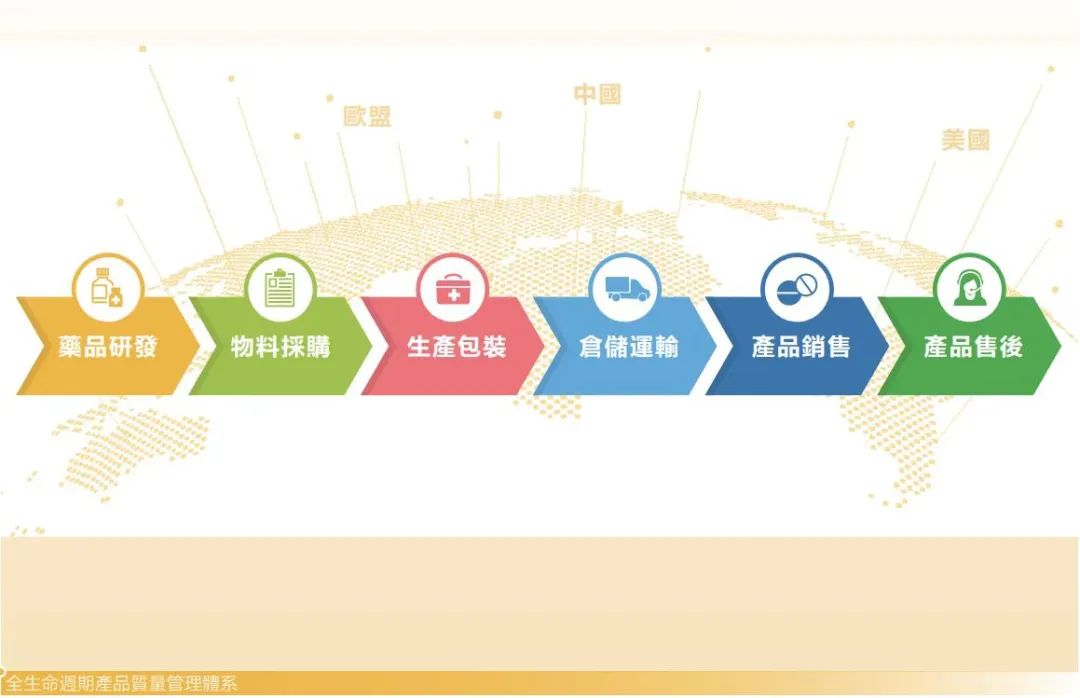
Sino Biopharm products are available to the global markets of China, the United States and the European Union. In order to comprehensively control quality risks in the global market and ensure quality compliance, the Group has established a quality management system covering the entire life cycle from drug development, material procurement, production and packaging, storage and transportation, product sales and after-sales, based on compliance with the regulations of each operation and market location, combined with international standards of pharmaceutical manufacturing quality management.
The Group has been building a quality culture in terms of quality awareness, management philosophy, operational standards and knowledge dissemination, etc. In 2021, the training coverage of personnel related to product quality control reached 100%. At the same time, the Group continues to improve the whole life cycle pharmacovigilance management model to identify potential product quality risks and deal with them in a timely manner to ensure public drug safety.
Sino Biopharm continues to strengthen the construction of responsible marketing system and conducts marketing training from time to time for executives, marketing and marketing personnel in different regions to strengthen employees' awareness of compliance marketing and enhance their service capabilities to ensure the provision of truthful and rigorous academic and product information, while ensuring the safety of patients' medication.
2、Information security and privacy protection
Sino Biopharm attaches great importance to information security and privacy protection of customers and partners, as well as the safety of drug use and privacy protection of subjects, and carries out safety evaluation of clinical drug trials on the basis of effective management of all operational aspects of clinical trials, firmly safeguarding the safety of drug use of subjects, while ensuring that personnel in all aspects do not have access to personal data of subjects. In 2021, the Group had no privacy breach or information security incident.

3、Diversified talent structure and younger reserve
Sino Biopharm attaches importance to staff training and development, and constantly improves the construction of talent layout. 2021, the Group's male to female ratio is of 53.99% and 46.01%, respectively, and the Group's female ratio in senior management takes up 30%. At the same time, the Group attaches importance to the reserve of young talents. Under the model of combining the old and the new, more and more young people get the opportunity to be promoted to management positions, and in 2021, the number of employees under 40 years old among the middle management of the Group will account for nearly 70%.
At the same time, the Group attaches importance to the cultivation of talents, through different ways such as specific training, performance coaching, rotation, secondment and attachment, combined with the systematic cultivation of member enterprises' organizational innovation centers, to help each employee improve their comprehensive ability and achieve their career planning goals.
4、 Shoulder responsibility, take part in public welfare
Sino Biopharm adhere to the values of "for the country, for the people and for the enterprise", take the initiative to assume social responsibility and actively participate in social welfare. In 2021, Sino Biopharm public welfare investment totaled 54,957,600 yuan, the total time invested in public welfare for 60438 hours.
In 2021, Sino Biopharm contributed to disaster relief, epidemic fighting, rural revitalization, education donation and charity in various aspects. For example, the Group donated more than 10 million yuan in total value of donations and materials to help with flood rescue and post-disaster reconstruction; it deeply participated in community epidemic prevention and control and took the initiative to donate materials to epidemic areas, with a total donation and materials of 1,844,400 yuan for the year against epidemics, etc.
| Focus on low carbon development and environmental protection, build a green home together
Adhering to the main vision of "actively responding to the national dual-carbon target and leading the low-carbon transformation of the industry chain", in FY 2021, Sino Biopharm's greenhouse gas emissions per million yuan of revenue were 11.52 tons of carbon dioxide equivalent, the comprehensive energy consumption per million yuan of revenue was 20.59 MWh, and the renewable energy usage of member enterprises reached 2906.70 MWh, an excellent level in the industry.
The Group continued to increase investment in environmental protection, and the total investment in environmental protection of the Group in 2021 reached more than 93.5 million RMB, an increase of nearly 5% compared with 2020. At the same time, we will take multiple measures to continuously promote energy conservation and emission reduction, energy structure improvement and new energy utilization, and strive to minimize the impact on the environmental climate caused by the production and operation of the Group and its member enterprises. Priority will be given to energy-saving equipment, and energy efficiency will be continuously improved and greenhouse gas emissions will be reduced by promoting energy-saving technology renovation, eliminating outdated energy-consuming equipment and expanding the use of new energy scenarios such as photovoltaics.

Water Recycling Facility in CTTQ
Sino Biopharm has incorporated "green development" into its corporate DNA and built a "green factory". The member company CTTQ actively responded to the national "Green Development Plan of the Industrial Sector" and was awarded the title of "Green Factory" at the national level after a rigorous review by third-party organizations with high standards and multiple dimensions.
In the future, Sino Biopharm will continue to invest in building scientific and technological innovation capabilities, promoting global communication and cooperation, and working together to overcome the public health problems caused by the epidemic. We will also be committed to providing the best and latest pharmaceutical and health services, to be the leader in China's pharmaceutical manufacturing industry, the innovator in the specialty medical industry, and the pioneer in the big health business, and to contribute to the pursuit of a better life for human beings.