News
CTTQ of Sino Biopharm's Anlotinib Compound Patent Wins China Patent Gold Award
Release time:2022-08-09
Recently, China National Intellectual Property Administration announced the decision of the 23rd China Patent Award, and the compound patent of Anlotinib, a new class 1 drug in the field of anti-tumor of Sino Biopharm CTTQ, "Spiro-substituted compounds as angiogenesis inhibitors" (Patent No. ZL200880007358.X), won the "China Patent Gold Award". This is one of the 30 gold awards selected from more than 2 million valid invention patents nationwide, and it is also the highest honor of Chinese patents for CTTQ again after 14 years. Before that, Magnesium Isoglycyrrhizinate Injection (Tianqing Ganmei), a key product in the field of liver disease, was awarded the 10th "China Patent Gold Award"
Mr. Tse, Eric S Y, Chief Executive Officer of Sino Biopharm and Chairman of CTTQ, said that innovative research and development have always been the core competitiveness of CTTQ, and strengthening intellectual property protection can effectively stimulate the innovative vitality of enterprises, and the company will benefit more patients by creating more high-quality intellectual property products.
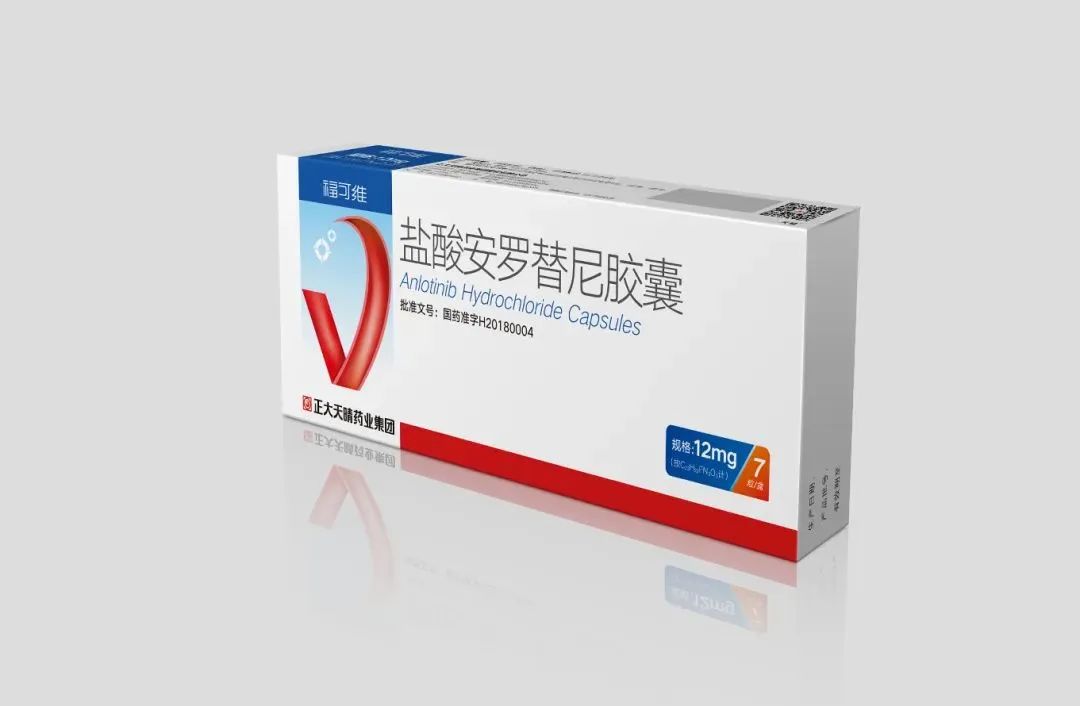
The patent ZL200880007358.X, which won the gold medal, is a compound patent for Anlotinib Hydrochloride Capsules. Through in-depth research and extensive screening of the compound structure, the invention introduces quinoline ring, indole group, cyclopropyl and fluorine substitution in the molecular structure, which improves the biological activity as well as kinase selectivity of the drug, introduces primary amino group on cyclopropyl and forms salt with hydrochloric acid to increase the water solubility and improves the bioavailability of the drug. In this year's Patent Award, another patent of Anlotinib, "Method and use of quinoline derivatives for the treatment of soft tissue sarcoma and pharmaceutical compositions for the treatment of soft tissue sarcoma" (Patent No. ZL201580026815.X), won the "China Patent Excellence Award". In addition to the award-winning patent, CTTQ has also laid out a number of patent applications around Anlotinib, forming a three-dimensional protection pattern with compound patent as the core and formulation, use, crystal form and combination drug as the peripheral protection.

China Patent Award, jointly selected by China National Intellectual Property Administration and the World Intellectual Property Organization, is the highest award in the field of patents in China. Winning the highest honor of China Patent twice is not only a fruitful achievement of the company in the field of innovation and R&D, but also a great affirmation of the harvest of intellectual property work.
It is known that CTTQ has set up the intellectual property strategy as early as twenty years ago, and now has an intellectual property management team consisting of more than 30 full-time employees. The team members are all with master's degree or above from nation's double first-class colleges and universities, and they help the innovation and development of enterprises through professional intellectual property services. Especially in terms of patent layout, in order to escort new products to market, CTTQ has built an international patent layout model, and has carried out patent layouts in more than 60 countries or regions around the world, including China, the United States, Britain, France, Germany, Japan and Korea. By the end of June 2022, there were 2402 valid patents and applications in total, including 2291 invention patents and 873 valid authorizations. The company also actively undertakes key projects such as the National Intellectual Property Demonstration Enterprise and the National Patent Operation Pilot Enterprise to continuously improve the quality of intellectual property output and strengthen the ability to implement intellectual property strategies.

The company is a core company of Sino Biopharm, a Hong Kong-listed company, and a well-known enterprise in the field of anti-tumor and liver disease in China, ranking 15th in the list of China's top 100 pharmaceutical industries, with more than 30 products with annual sales of over 100 million RMB. Anlotinib is a new structured small molecule multi-target tyrosine kinase inhibitor, which has been approved by the State Drug Administration for 5 indications. In the newly released 2022 CSCO guidelines, Anlotinib has been authoritatively recommended by the guidelines in six therapeutic areas, including non-small cell lung cancer, small cell lung cancer, thyroid cancer, soft tissue sarcoma, esophageal squamous cancer and kidney cancer, demonstrating the company's strength of "the light of Chinese original research".